Strategizing from 7 cities across the globe
Human-Grown Heart Valves: A Promising Breakthrough to the Treatment of Valvular Heart Diseases
Anabelle Rizkallah


Introduction
Our body revolves around a central pump, the heart, which circulates blood through an extensive network of blood vessels reaching all our organs. With every beat, the heart steadily pumps out blood through its 4 chambers, with its valves acting as meticulous gatekeepers. A valve is a small flap of tissue at the exit of each heart chamber that can only open and close in a singular direction, ensuring the unidirectional flow of blood in our body’s circulation. When these valves falter, the heart’s harmonious rhythm is disrupted, giving rise to turbulence and inefficiency. Valvular heart diseases, whether congenital or acquired, have long posed challenges for patients, particularly children. Current surgical treatments involve replacing the defective valve with a mechanical or biological valve, often requiring multiple subsequent surgeries in children, as these valves aren’t capable of growing on their own. Today, the field of medicine stands on the brink of a transformative breakthrough, as researchers are making heart valves from human stem cells. These human-grown heart valves could potentially spare children from additional surgeries, completely redefining the treatment of valvular disease.
Background:
Valvular heart diseases correspond to any defects in one or more of the heart’s four valves, preventing them from opening and closing properly. Thus, the passage of blood through these valves becomes increasingly challenging, forcing the heart to exert more effort to keep a sufficient amount of blood flowing in the right direction. The type and magnitude of symptoms differ depending on the type of valvular disease; they can range from fever and chest pain to more severe symptoms such as heart failure, stroke, blood clots, or even death due to sudden cardiac arrest (American Heart Association, 2024).
Valvular heart diseases can be classified into stenosis, regurgitation, and atresia. In stenosis, the valve openings are narrowed and blood flow is constricted, while in regurgitation, the valves are leaky and thus allow backflow of blood. Atresia, on the other hand, is a congenital disorder (occurring at birth) characterized by the incomplete formation of the valves. A prolapse is also a type of abnormality where the valve bulges into the left atrium instead of closing properly, but it is mostly benign and causes minimal trouble for the patient. The most common valvular disease worldwide is Rheumatic Heart Disease, affecting an estimated 55 million people worldwide and claiming approximately 360,000 lives each year (World Health Organization, 2025). It is caused by an anti-inflammatory response to strep throat and leads to rheumatic fever and damage to the heart valves. This specific kind of valvular disease is more prominent in younger people, where it often occurs in children ages 5 to 15 (John Hopkins Medicine, n.d.). Patients with this illness usually suffer from chest pain, shortness of breath, and swelling, and may require surgery to replace the damaged heart valves in severe cases.
Conventional Techniques:
One type of replacement for damaged valves is mechanical valves. They are composed of strong metals (often titanium) and carbon, making them sturdy enough to last for years. Despite this advantage, mechanical valves come with the risk of blood clots, which puts the patients at risk of strokes. Thus, patients who undergo surgery for this kind of replacement have to take anticoagulant medicines such as warfarin to prevent blood clotting and ensure a thinner and smoother flow of blood. Since these medications are also associated with bleeding, patients have to do regular blood tests to monitor progress and adjust dosages accordingly. Mechanical valves are most favorable for patients below 50 for an aortic valve and below 65 for a mitral valve (Professional, 2025, sec. 4).
Another class of valves is the bioprosthetic valves, which are made of biological materials, particularly porcine (pig) and bovine (cow) tissue, due to the similar structure between the cardiovascular systems in these animals and humans. Unlike mechanical valves, they are characterized by better hemodynamics (blood flow), which spares the patients from the complexity of anticoagulation therapy. This advantage, however, is countered by the shorter durability of these valves. This process of structural valve deterioration includes leaflet wear and tear, disruption, flail leaflet, leaflet fibrosis (accumulation of excess fibrous connective tissue), and/or calcification and thickening (Pibarot et al., 2022). There is also the issue of incompatibility that arises with xenografts, which also impedes the treatment and might result in the destruction of the heart tissue. Hence, patients who undergo this type of treatment are at higher risk of reoperation than those who choose mechanical valves. Such features make bioprosthetic valves more suitable for people above 65, who prioritize function over longevity at this age. This is the general trend, but the choice of valves is determined on a case-by-case basis.
The implementation of either type of valve is complicated and necessitates surgery. Mechanical valve replacement requires a traditional open-heart surgery that involves opening the chest and cutting the breastbone to reach the valve area. During the replacement, the heart needs to be temporarily stopped, so a heart-lung bypass machine artificially circulates blood around the patient’s body during the operation (British Heart Foundation, n.d.). Bioprosthetic valve replacement can follow the same track as the former (through traditional open-heart surgery) or can be done by transcatheter valve replacement. This is a less invasive technique where the new valve is inserted through certain insertion sites (mainly transfemoral/ groin or transapical/ribs ) without taking out the old, damaged valve. Once the new valve is expanded, it pushes the old valve leaflets out of the way, and the tissue in the replacement valve takes over the job of regulating blood flow (American Heart Association, 2024). Although it might be a safer option than open heart surgery in some cases, it also has its own risks of failure (if the valve slips out, for instance), which will force the patient to undergo another reparatory surgery. In addition to being expensive (ranges from $50,000 to $200,000 in the US (Team, 2024) ), these surgeries are physically and emotionally taxing for the patients and do not ensure guaranteed results, which is why recent efforts in the field of cardiac medicine have been focused on engineering valves made from human stem cells as a new treatment method.
Download the full document
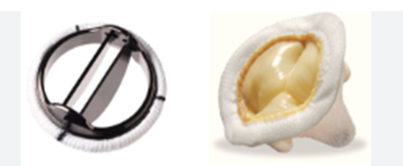

Figure 1: Mechanical vs bioprosthetic valves. Left: Mechanical mitral valve. Right: Bioprosthetic mitral valve.
Human-Grown Heart Valves
Researchers have shifted their focus towards deriving heart valves from human stem cells, since these cells will not be rejected by the host’s immune system and will be able to grow and adapt to the patient’s physiology, thus providing a permanent treatment. However, the differentiation of heart valve cells from human induced pluripotent stem cells (hiPSCs) is a complex process. Research began with extensive observations of heart valve cells, especially embryonic ones, to record structure, function, mode of replication, and signaling pathways. This has been facilitated by single RNA sequencing and modern imaging techniques available such as X-rays, Computed Tomography (CT), Magnetic Resonance Imaging (MRI), Echocardiograms, Positron Emission Tomography (PET)/single-photon emission computed tomography (SPECT), and Electroanatomical Mapping (Counseller & Aboelkassem, 2023). After collecting relevant information about the transcriptional genes and molecules involved, several experiments were conducted to try and replicate the differentiation process. A study conducted by doctors in the Cardiology department of Union Hospital, Fujian Medical University, China, identified CD144+ cells and CD144– cells as hiPSC-derived valve endothelial-like cells and hiPSC-derived valve interstitial-like cells, respectively (Cai et al., 2024). Samples of hiPSCs were cultured in nutrient-rich media, also incorporating other molecules needed for heart valve cell differentiation, such as VEGF-165, forskolin… (Cai et al., 2024). 6 days after the start of the experiment, the CD144 + and CD144- cells were isolated and their sequences were compared to data obtained from the Gene Expression Omnibus (GEO) database. The markers carried by cells were analyzed using software such as FindMarkers (Cai et al., 2024). Results showed that day 6 cells are divided into 2 groups: the first cluster, which constituted ≈70% of the population, highly expressed endothelial cell markers (CD31, CD144, and KDR), whereas the other cluster, which forms the remaining 30% of the population, highly expressed interstitial cells markers (COL1A1, COL3A1, and BAMBI ). These cells showed high similarity in structure and function to the normal cardiac cells, marking significant progress towards the final goal. Although this study allowed the production of functional endothelial and interstitial valvular heart cells, its results are inconclusive as they are inconsistent (Cai et al., 2024).
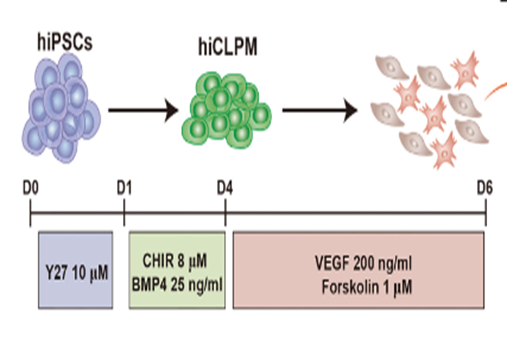
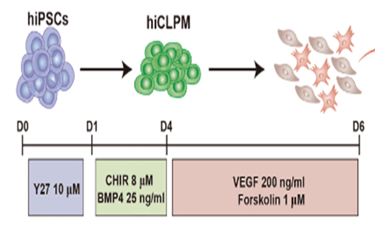
Figure 2: Generation of CD144+ cells and CD144- cells from hiPSCs. Schematic of the differentiation protocol.
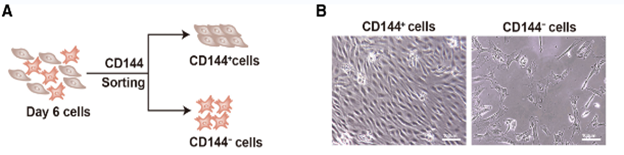
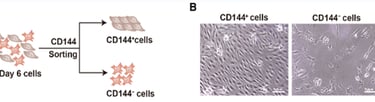
Figure 3: CD144+ cells are endothelial cells, and CD144– cells are interstitial cells. A: Schematic of CD144 magnetic beads sorting. B: Morphology of CD144+ cells and CD144– cells.
Another research study, in which doctors from the Icahn School of Medicine collaborated with doctors from Stanford School of Medicine, focused on the addition of the bone morphogenetic protein 10 (BMP-10) to induce pluripotent stem cells (iPSCs) to enhance the expression of endocardial-specific genes (Liu et al., 2023). This included the up-regulation of transcription factors such as NKX2.5, GATA4, and GATA5. After culturing the iPSCs with BMP-10, the researchers added BMP2 (100 ng/mL), TGFβ2 (0.3 ng/mL), and bFGF (10 ng/mL), powerful growth factors used to induce an endothelial-to-mesenchymal transition (EndMT), which is an essential process in valve formation. The results showed that a subpopulation of the culture underwent EndMT to produce valvular interstitial cells, while others escaped it, transforming into valvular endothelial cells (Liu et al.,2023). This is increasingly similar to what happens in the natural formation of heart valves, making this study a promising breakthrough. However, one of the drawbacks faced was the limited yield of cells produced, ranging between 15% and 30% only (Liu et al., 2023).
Due to the limitations mentioned above, this therapy has not yet entered any clinical trials. However, research is still ongoing to build upon these discoveries in pursuit of more satisfactory results.
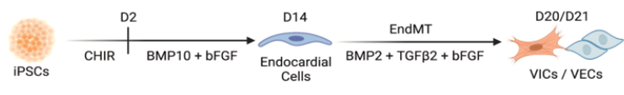
Figure 4: Generation of endocardial populations from iPSCs. Schematic of differentiation timeline
Ethical concerns:
Although human stem cell therapies represent a significant advancement in the field of medicine, promising to bring relief to many cardiac patients, they continue to raise ethical concerns. One of the concerns involves the source of the stem cells, particularly embryonic stem cells. Since using embryonic cells will deprive the embryo of developing into a human being, many argue that it stands against ethical and moral values to deny a potential human being from existence (The Hastings Center, 2023). Opinions on this matter vary depending on each individual’s moral and religious beliefs, and there are ongoing debates on whether the sacrifice of these embryonic cells is justified for the greater good of society. Another ethical dilemma is the usage of stem cell technology for non-therapeutic purposes. This entails cloning and genetic modification. Many people find this alarming in the sense that it might erase individuality and potentially change societal norms, especially when it comes to producing and rearing children (Elliot, n.d.) By enabling control over the gender and genetic traits of embryos—and even making single parenting possible—modern reproductive technologies are revolutionizing the concept of the traditional family. This shift has created conflict between supporters and opponents of these advances. The dichotomy created has made it difficult to develop a satisfactory policy regarding the funding conditions of embryonic stem cells by governments. However, scientists and policymakers are joining their efforts to implement stem cell therapy while attempting to avoid potential ethical concerns.
Conclusion:
The advancement of research in stem cell engineering holds the promise of a new therapy that will alleviate the suffering of cardiac patients, enhancing their quality of life. Patterns regarding the genes involved in the differentiation process are already emerging, which is a crucial part of the process. Although there are still gaps concerning limited yield and inconsistent results in the literature, scientists are tackling these issues and making significant progress in their studies. With adequate support, this field is guaranteed to prosper, leading to treatments that are effective and safe for clinical application. It is imperative to address the limitations and concerns surrounding this issue to reinforce the development of human-grown heart valves and contribute to helping cardiac patients all over the world.
References
American Heart Association. (2024). What is TAVR? (TAVI). American Heart Association. https://www.heart.org/en/health-topics/heart-valve-problems-and-disease/understanding-your-heart-valve-treatment-options/what-is-tavr
American Heart Association. (2024, 5). Risks for Heart Valve Problems. https://www.heart.org/en/health-topics/heart-valve-problems-and-disease/heart-valve-disease-risks-signs-and-symptoms/risks-for-heart-valve-problems
British Heart Foundation. (n.d.). Replacement heart valves. British Heart Foundation. https://www.bhf.org.uk/informationsupport/heart-matters-magazine/medical/replacement-heart-valves#:~:text=How%20are%20replacement%20heart%20valves,your%20body%20during%20the%20operation.
Cai, Z., Zhu, M., Xu, L., Wang, Y., Xu, Y., Yim, W. Y., Cao, H., Guo, R., Qiu, X., He, X., Shi, J., Qiao, W., & Dong, N. (2024). Directed differentiation of human induced pluripotent stem cells to heart valve cells. Circulation, 149(18), 1435–1456. https://doi.org/10.1161/circulationaha.123.065143
Elliot, J. (n.d.). ETHICAL CONSIDERATIONS IN STEM CELL RESEARCH. https://www.bioethics-singapore.gov.sg/files/publications/bac-publications/ethical-considerations-in-stem-cell-research.pdf
Johns Hopkins Medicine. (n.d.). Rheumatic Heart Disease. Johns Hopkins Medicine. https://www.hopkinsmedicine.org/health/conditions-and-diseases/rheumatic-heart-disease
Kuhns, L. (2024, 2). Prevalence of Childhood Rheumatic Heart Disease Increased Over the Past 30 Years. https://www.rheumatologyadvisor.com/news/childhood-rhd-prevalence-increased-1990-to-2019/?utm_source=chatgpt.com
Liu, C. Z., Prasad, A., Jadhav, B., Liu, Y., Gu, M., Sharp, A. J., & Gelb, B. D. (2023). Feeder-free generation and characterization of endocardial and cardiac valve cells from human pluripotent stem cells. iScience, 27(1), 108599. https://doi.org/10.1016/j.isci.2023.108599
Pibarot, P., Herrmann, H. C., Wu, C., Hahn, R. T., Otto, C. M., Abbas, A. E., Chambers, J., Dweck, M. R., Leipsic, J. A., Simonato, M., Rogers, T., Sathananthan, J., Guerrero, M., Ternacle, J., Wijeysundera, H. C., Sondergaard, L., Barbanti, M., Salaun, E., Généreux, P., . . . Leon, M. B. (2022). Standardized definitions for bioprosthetic valve dysfunction following aortic or mitral valve replacement. Journal of the American College of Cardiology, 80(5), 545–561. https://doi.org/10.1016/j.jacc.2022.06.002
Professional, C. C. M. (2025, March 19). Heart valve replacement. Cleveland Clinic. https://my.clevelandclinic.org/health/treatments/23966-heart-valve-replacement
Team, R. (2024, February 1). The true cost of heart valve replacement. https://www.resolvemedicalbills.com/blog/the-true-cost-of-heart-valve-replacement
The Hastings Center. (2023, September 11). Stem cells - the Hastings Center. https://www.thehastingscenter.org/briefingbook/stem-cells/
World Health Organization [WHO]. (2025, 1). Rheumatic heart disease. World Health Organization (WHO). https://www.who.int/news-room/fact-sheets/detail/rheumatic-heart-disease
