Strategizing from 7 cities across the globe
Gestational Diabetes in Pregnancy: Understanding the Risks and Promoting Effective Management
hala ramadan, toros torossian


Introduction
For many women, a positive pregnancy test brings joy, but for those with diabetes, it can also bring a wave of anxiety. Diabetes is a common chronic medical condition characterized by the body's inability to spontaneously and adequately regulate blood glucose levels. During pregnancy, the placental hormones augment glucose production, which can overwhelm maternal mechanisms to cope with this new glucose load, eventually disrupting maternal metabolism. The excess glucose then passes through the placenta, affecting the baby’s health, increasing the risks of miscarriage and fetal anomalies, and compromising fetal growth potential [1-3]. When looking at diabetes during pregnancy, it can take two forms: (1) pre-gestational diabetes mellitus (PGDM), which is a condition existing before pregnancy, requiring ongoing modifications of appropriate management. (2) gestational diabetes mellitus (GDM), which develops during pregnancy and usually resolves after childbirth [1].
From a historical standpoint, the past two decades have witnessed the rise in GDM, affecting 14% of all pregnancies worldwide [4]. Notably, the Middle East and North Africa (MENA) region has the highest prevalence, with 27.6% of pregnancies affected by GDM [4]. Despite this growing prevalence, many women remain unaware of the risks associated with GDM [5]. Unlike PGDM, which is diagnosed before pregnancy and requires prior management, GDM develops during pregnancy, with its incidence increasing throughout gestation, reaching 26% in the second trimester and 33% in the third trimester [6]. Given its rising prevalence and the lack of awareness surrounding it, this review will primarily focus on GDM.
Research has shown that GDM poses significant short-term and long-term health concerns, both for the mother and the newborn infant [7]. For the newborn infant, GDM can lead to physiological complications, birth trauma, and impaired neural development [1-3, 8-10]. For the mother, it increases the risk of hypertension and mental health issues [11-14]. Moreover, it can also increase the potential for chronic diseases, such as developing lifelong diabetes and cardiovascular problems among both mother and offspring [3, 10, 15-17].


Download the full document
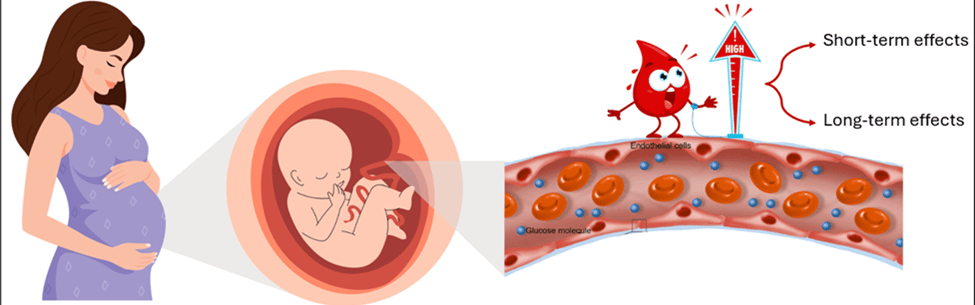
Figure 1: Elevated maternal blood glucose crosses the placenta, exposing the fetus to high glucose levels, which can lead to both short-term and long-term health effects
Given these risks to both mother and child, managing blood glucose levels during pregnancy is essential. Early diagnosis and intervention are critical not only to manage symptoms and prevent complications but also to reduce the likelihood of developing long-term chronic conditions. As a result, regular checkups and screenings are essential to detect GDM at an early stage. This detection will therefore allow for more timely interventions and treatment of the condition, such as lifestyle modifications, changes in dietary habits, increased physical activity, and weight management. [10, 18].
Despite the established management strategies for GDM, no universally effective treatment method ensures optimal outcomes for all pregnant individuals. In addition, the long-term impact of different treatment strategies on both maternal and offspring health remains insufficiently researched, warranting the need for further testing into individualized and targeted interventions.
This review aims to raise awareness of maternal and neonatal risks related to GDM and to highlight effective strategies for managing GDM, reassuring pregnant women that regular prenatal visits and adoption of a healthy lifestyle can ensure positive outcomes.
Prenatal and Postnatal Risks for the Neonate
GDM is a medical condition that can lead to adverse neonatal outcomes. Although this disorder is first discovered during pregnancy, many women might have a silent type II Diabetes Mellitus (DM) before conception. High maternal blood sugar levels during embryogenesis (early pregnancy) in women with GDM are associated with an increased risk of congenital anomalies [19]. Hence, if GDM is not recognized and managed properly, offsprings of diabetic mothers are at an increased risk of experiencing both short and long-term sequelae. Uncontrolled glucose levels can even lead to stillbirth, which is the death of the fetus in the uterus after 20 weeks of pregnancy. A recent study has shown that the risk of stillbirth in pregnant women with GDM is 4 to 5 times higher than in women with normal pregnancies [3, 4]. As pregnancies progress, the risk of stillbirth becomes increasingly significant [5]. Additionally, mothers are also at a higher risk of developing preeclampsia, which is a condition characterized by high blood pressure and proteinuria (presence of protein in the urine), necessitating preterm delivery, ergo increasing the risk for neonatal prematurity with all its related complications.
Moreover, maternal hyperglycemia causes excess glucose to cross the placenta, exposing the fetus to high glucose levels. In response, the fetal pancreas produces more insulin to combat the excess glucose. Fetal insulin has a growth hormone-like action, promoting the intrauterine growth of the fetus. First, metabolic and growth-related complications arise when elevated insulin levels promote the storage of glucose as fat, leading to excessive fetal growth, known as macrosomia, which increases the risk of birth trauma. [3, 20]. Additionally, newborns with mothers with GDM are also more prone to develop hypoglycemia immediately after birth, as their bodies continue producing high amounts of insulin even after the excess glucose supply from the mother is no longer available [21, 22]. Second, respiratory and oxygenation issues become more common. High insulin levels make neonates more susceptible to postpartum respiratory problems due to poor secretion of the surfactant, a critically needed substance for lung function [23].
Rapid fetal growth demands significant energy, which can lead to lower oxygen levels in the fetus [24]. In response, the body produces more erythropoietin (EPO), a hormone that stimulates red blood cell (RBC) production to compensate for low oxygen levels [24]. These RBCs contain hemoglobin, which is broken down at the end of their lifespan, producing bilirubin as a byproduct. After birth, the sudden availability of oxygen through respiration reduces EPO levels, leading to the breakdown of excess RBCs. This breakdown further increases bilirubin levels, which are toxic in high amounts. Under normal conditions, bilirubin is processed by the liver and excreted into the intestine via bile. However, in neonates, the liver is still immature, limiting its ability to eliminate bilirubin from the body efficiently. As a result, some bilirubin is reabsorbed from the intestine due to the enterohepatic circulation, which further raises its levels. Excessive bilirubin accumulation in the bloodstream can lead to neonatal jaundice, which is the yellowish discoloration of the sclera and skin of the baby.
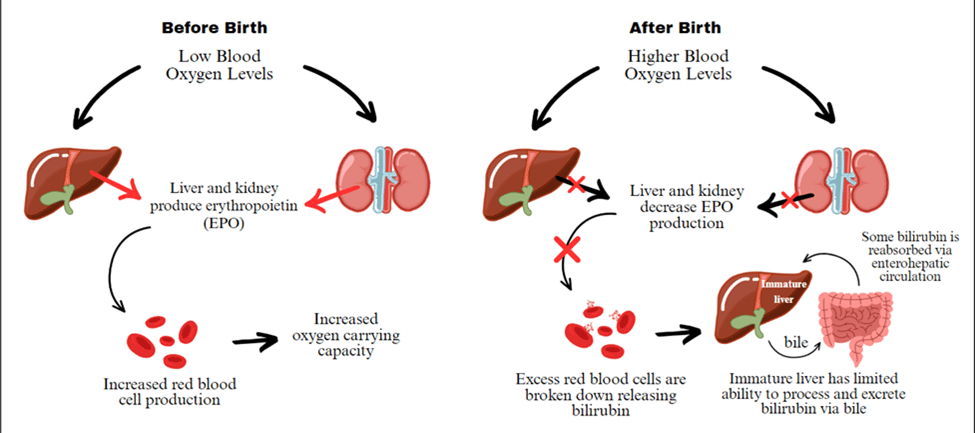
Figure 2: Mechanisms Behind Red Blood Cell Turnover and Bilirubin Processing Before and After Birth
In the long term, insulin itself has pro-inflammatory effects, creating an unfavorable environment for fetal growth. In a typical pregnancy, specialized immune cells called T lymphocytes help maintain an anti-inflammatory state for proper maternal-fetal immune interactions and fetal development [1]. However, in diabetic pregnancies, elevated levels of glucose induce the fetus into a pro-inflammatory state, where the immune system becomes overactive in the “alert” mode, as if it is constantly fighting something harmful, even when there is no real threat [1]. This inflammatory response increases oxidative stress, further complicating fetal development [25].
Maternal hyperglycemia can profoundly impact fetal brain development. High glucose levels can disrupt neural stem cell differentiation and thus alter brain structure [26, 27]. These changes trigger epigenetic modifications, such as alterations in how genes are marked and regulated, which affect the expression of genes responsible for neuron formation and differentiation [27, 28]. This disruption can lead to the premature differentiation of neural stem cells, causing neurons to migrate incorrectly into abnormal layers of the brain’s cortex [27, 28]. Simultaneously, the density of Purkinje and granular cells is reduced, potentially leading to a smaller fetal cerebellum. These structural and epigenetic modifications, coupled with a pro-inflammatory state, contribute to neurobehavioral disorders in offspring of mothers with GDM. Misplaced neurons may impair cognitive and executive functions, while an underdeveloped cerebellum can affect motor coordination and emotional regulation. Additionally, the pro-inflammatory environment disrupts neurotransmitter balance, increasing the risk of psychosocial disorders such as autism spectrum disorder, ADHD, and schizophrenia [1, 3, 9, 11, 29].
Beyond structural changes, epigenetic mechanisms also contribute to the long-term effects of GDM on fetal health, increasing the risk of cardiovascular disease and type 2 diabetes later in life [3, 17]. High blood sugar levels in GDM pregnancies lead to abnormal blood vessel growth in the fetus, causing weaker connections between cells and impairing blood vessel function [30]. Recent studies suggest that microRNAs (miRNAs) play a crucial role in these processes. In particular, elevated levels of miR-101 in babies of mothers with GDM suppress the activity of EZH2, a gene regulator essential for proper blood vessel development [17]. This disruption can have lasting effects, even after blood sugar levels return to normal. This phenomenon, known as metabolic memory, means that early exposure to high blood sugar can leave lasting imprints on the child’s health, making them more susceptible to future health issues like diabetes and heart disease [17].
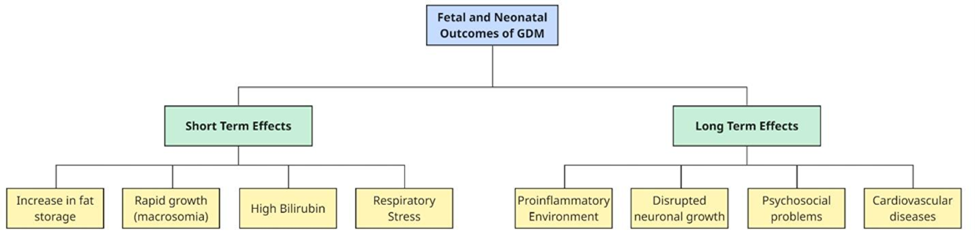
Figure 3 – Short-term and Long-term effects of GDM on the neonate
Prenatal and Postnatal Risks for the Mother
During a normal pregnancy, insulin resistance naturally occurs in the late second trimester, as maternal cells become less responsive to insulin [31]. This is a physiological adaptation that ensures an adequate amount of glucose is available for the developing fetus. In response, the maternal pancreas compensates by producing more insulin. While this compensatory mechanism helps most women maintain normal blood glucose levels, it becomes insufficient when insulin resistance is too pronounced [31]. In such cases, the pancreas is unable to produce enough insulin to overcome the resistance, which elevates blood glucose levels and the onset of GDM [31].
Studies have shown that insulin resistance alters the metabolism of both carbohydrates and lipids [32]. In women with GDM, this often leads to dyslipidemia characterized by abnormal blood lipid levels, including high LDL, elevated triglycerides, and low HDL levels. While lipid levels normally rise during pregnancy to support steroid hormone production and fetal development, these changes typically resolve after delivery and are not pathological. However, in GDM, the lipid profile may become more severely disrupted, potentially contributing to vascular dysfunction. In short, oxidized LDL damages endothelial cells by reducing nitric oxide production, which will in turn promote inflammation and impair normal blood vessel relaxation [33, 34]. At the same time, elevated triglycerides and reduced HDL protective effects further worsen endothelial dysfunction, making blood vessels more susceptible to damage [33].
Endothelial dysfunction is believed to be the primary driver of preeclampsia [35]. Recent research has shown that bioactive factors disrupt normal blood vessel function, leading to widespread vasoconstriction and increased vascular resistance, which increases blood pressure [33, 34, 36]. This heightened pressure affects multiple organs. In the kidneys, endothelial damage impairs the glomerular filtration barrier, resulting in increased permeability and the abnormal leakage of proteins into the urine, a condition known as proteinuria. This reflects early signs of renal impairment, as the kidneys normally retain proteins and prevent their excretion. In the brain, elevated blood pressure can cause vision disturbances, headaches, and seizures [34]. The liver is also affected, contributing to red blood cell breakdown and low platelet levels [34].
As these complications progress, severe cases may require preterm delivery to protect both the mother and fetus. Additionally, women with GDM face a higher likelihood of requiring a cesarean section, partly due to complications like preeclampsia. Studies show that the C-section rate is 40.3% in women with GDM, compared to 29.7% in those without [37].
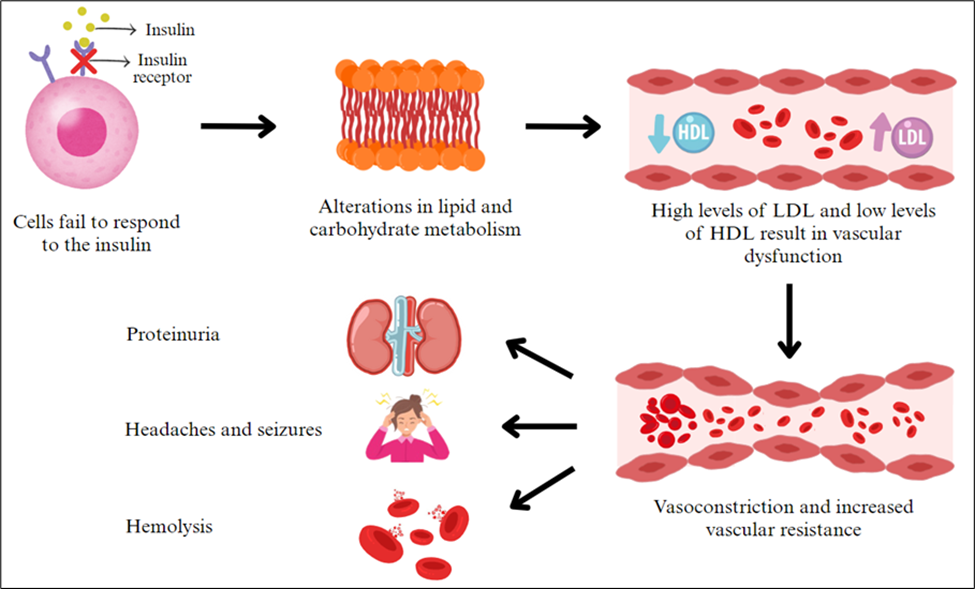
Figure 4 - Insulin resistance-mediated vascular dysfunction and its downstream complications
Beyond vascular complications, GDM is also associated with psychosocial burdens, often leading to psychological strain, confusion, and frustration [11, 13]. Pregnant women with GDM are twice as likely to develop depression before birth and 2 to 4 times more likely to experience postpartum depression compared to those without GDM [11]. A study by Hui et al. [13] identified three primary sources of anxiety and depression in these women:
· The first source is stress from the GDM diagnosis and perception of a high-risk pregnancy.
· The second source is the challenge of managing GDM, particularly dietary control.
· The third source is anxiety about potential complications for both mother and infant.
Among these, concerns about maternal and infant complications emerged as the leading source of anxiety and stress [13].
Additionally, maternal stress is a significant risk factor for adverse pregnancy and birth outcomes [12]. It disrupts various physiological systems, including the autonomic nervous and endocrine systems. Anxiety and depression are believed to alter maternal hormone levels, triggering the hypothalamic-pituitary-adrenal axis and increasing cortisol release. This, in turn, exacerbates insulin sensitivity and amplifies the negative effects of GDM. The activation of the sympathetic nervous system in hypertensive patients further disrupts circadian rhythms, leading to poor sleep quality [12]. Combined with societal stigma, these factors can reinforce feelings of shame and self-blame, ultimately impacting the mental health of individuals with GDM [14].
As previously mentioned, hormonal changes during pregnancy naturally lead to increased insulin resistance, and pancreatic β-cells compensate by increasing insulin secretion [3]. However, this adaptation induces cellular stress. An animal study examining the impact of multiple pregnancies found that β-cells from multiparous individuals lost their ability to proliferate and exhibited signs of aging and senescence [38]. This decline in function was linked to pregnancy and childbirth, increasing their likelihood of developing type 2 diabetes mellitus postpartum [3].
Other studies have found similar associations, showing that women with a history of GDM are nearly 10 times more likely to develop type 2 diabetes mellitus (T2DM) compared to those who had normal blood glucose levels during pregnancy [3, 10, 16]. Furthermore, recent research indicates that GDM is associated with a higher predicted risk of heart disease 10 to 30 years after delivery compared to pregnancies without complications [3, 10, 15]. These findings highlight the need for long-term cardiovascular monitoring and preventive strategies for women with a history of GDM.
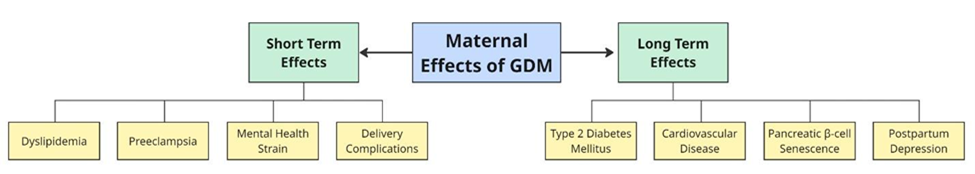

Figure 5 – Major short- and long-term complications of GDM on maternal health
Management of Gestational Diabetes Mellitus
Given the serious short-term and long-term consequences of GDM, effective management of blood glucose levels is essential to prevent complications. There are two main strategies for managing GDM: lifestyle modification and pharmacological therapy [1]. However, there is currently no universally accepted standard treatment. Therefore, a comprehensive approach is recommended, which includes educating the patient about managing pregnancy weight gain, maintaining blood glucose control, and participating in regular nutritional follow-ups [2].
Moreover, research has shown that 70–85% of GDM cases can be successfully managed through lifestyle changes and physical activity alone, with only 15–30% of patients requiring pharmacological intervention [2]. Nutritional modifications are central to lifestyle management. Specifically, carbohydrate intake should be limited to 40% of total calories, protein intake should make up 20%, and fats should comprise 40% of the daily caloric intake [1]. Instead of focusing solely on calorie restriction, healthcare providers often advise changes in food types and distribution. However, for obese pregnant women, a 30% reduction in caloric intake below the Dietary Reference Intakes (DRIs) may be recommended (Moreno-Castilla et al., 2013; American Diabetes Association, 2004).
Physical activity also plays an essential role in improving glycemic control. Pregnant women with GDM are typically encouraged to engage in moderate-intensity exercise, such as walking or prenatal yoga, for at least 30 minutes a day. Exercise enhances insulin sensitivity and contributes to better glucose regulation.
If high blood sugar levels persist after 10 to 14 days of nutritional and lifestyle adjustments, pharmacological therapy is usually initiated. This may include insulin therapy or oral antihyperglycemic agents such as metformin and glyburide [2]. Insulin is generally considered the safest option during pregnancy, as its large molecular size prevents it from crossing the placenta [1][2]. In contrast, metformin and glyburide are smaller molecules and have been shown to cross the placental barrier, reaching the fetus [48]-2. As such, there are ongoing concerns regarding their long-term safety for both the mother and the child. It is also important to note that metformin is not approved by the FDA specifically for the treatment of GDM [1].
In addition to conventional treatments, recent research has explored the use of probiotics. Supplementing pregnant women with GDM with probiotics has been shown to reduce the risk of neonatal hyperbilirubinemia by 74% (Zhang et al., 2019) as probiotics contribute to better glycemic control, improved lipid profiles, and reduced markers of inflammation and oxidative stress.
Lastly, postpartum follow-up is essential, as women with GDM have an elevated risk of developing type 2 diabetes and cardiovascular diseases later in life. It is recommended that patients undergo a 75g oral glucose tolerance test (OGTT) between 6 and 12 weeks after delivery to evaluate glucose metabolism and determine whether further management is necessary.
Conclusion
Gestational diabetes can lead to severe and potentially life-threatening maternal and neonatal consequences if not properly controlled. For the neonate, complications such as macrosomia, neonatal hypoglycemia, and long-term metabolic dysfunction are major concerns. For the mother, GDM increases the risk of obstetric complications and the later development of type 2 diabetes mellitus and cardiovascular disease. While current management strategies are effective in many cases, no universally optimal treatment approach exists. Outcomes can vary significantly between individuals, and the long-term effects of different treatment modalities on both maternal and offspring health remain inadequately understood. Universal screening between weeks 24 and 28 of pregnancy or earlier in high-risk individuals is essential for timely recognition and intervention. Maintaining fasting and postprandial glucose within normal levels is crucial to ensuring the health and safety of both mother and child. However, further research is needed to develop more personalized and targeted interventions that improve both short- and long-term outcomes for mothers and their children.
References
1. Maheshwari, A., et al., Neurological Abnormalities in Infants of Mothers with Diabetes Mellitus. Newborn, 2022. 1: p. 238-244.
2. Wang, H., et al., Maternal diabetes and the risk of feeding and eating disorders in offspring: a national population-based cohort study. BMJ Open Diabetes Res Care, 2020. 8(1).
3. Lee, J., N.K. Lee, and J.H. Moon, Gestational Diabetes Mellitus: Mechanisms Underlying Maternal and Fetal Complications. Endocrinol Metab (Seoul), 2025.
4. Wang, H., et al., IDF Diabetes Atlas: Estimation of Global and Regional Gestational Diabetes Mellitus Prevalence for 2021 by International Association of Diabetes in Pregnancy Study Group's Criteria. Diabetes Res Clin Pract, 2022. 183: p. 109050.
5. Vu, A., et al., Association of Type 2 Diabetes Risk Perception With Interest in Diabetes Prevention Strategies Among Women With a History of Gestational Diabetes. Diabetes Spectr, 2022. 35(3): p. 335-343.
6. Wang, Z., et al., Clinical analysis of diabetes in pregnancy with stillbirth. Medicine, 2023. 102(21): p. e33898.
7. Reece, E.A., The fetal and maternal consequences of gestational diabetes mellitus. J Matern Fetal Neonatal Med, 2010. 23(3): p. 199-203.
8. Feig, D.S., et al., Long-term Neurobehavioral and Metabolic Outcomes in Offspring of Mothers With Diabetes During Pregnancy: A Large, Population-Based Cohort Study in Ontario, Canada. Diabetes Care, 2024. 47(9): p. 1568-1575.
9. Garza-Martínez, M.J., et al., Maternal diabetes during pregnancy and offspring's risk of autism spectrum disorder: A systematic review and meta-analysis. J Psychiatr Res, 2025. 182: p. 100-115.
10. Nakshine, V.S. and S.D. Jogdand, A Comprehensive Review of Gestational Diabetes Mellitus: Impacts on Maternal Health, Fetal Development, Childhood Outcomes, and Long-Term Treatment Strategies. Cureus, 2023. 15(10): p. e47500.
11. Benton, M., S.A. Silverio, and K. Ismail, "It feels like medically promoted disordered eating": The psychosocial impact of gestational diabetes mellitus in the perinatal period. PLoS One, 2023. 18(7): p. e0288395.
12. Hayase, M., M. Shimada, and H. Seki, Sleep quality and stress in women with pregnancy-induced hypertension and gestational diabetes mellitus. Women Birth, 2014. 27(3): p. 190-5.
13. OuYang, H., et al., Associations between Gestational Diabetes and Anxiety or Depression: A Systematic Review. J Diabetes Res, 2021. 2021: p. 9959779.
14. Sun, S., et al., Experiences of stigma, psychological distress, and facilitative coping among pregnant people with gestational diabetes mellitus. BMC Pregnancy and Childbirth, 2023. 23(1): p. 643.
15. Venkatesh, K.K., et al., Hypertensive disorders of pregnancy and gestational diabetes mellitus and predicted risk of maternal cardiovascular disease 10-14 years after delivery: A prospective cohort. Diabet Med, 2025: p. e15516.
16. Vounzoulaki, E., et al., Progression to type 2 diabetes in women with a known history of gestational diabetes: systematic review and meta-analysis. Bmj, 2020. 369: p. m1361.
17. Słupecka-Ziemilska, M., P. Wychowański, and M. Puzianowska-Kuznicka, Gestational Diabetes Mellitus Affects Offspring's Epigenome. Is There a Way to Reduce the Negative Consequences? Nutrients, 2020. 12(9).
18. Ringholm, L., P. Damm, and E.R. Mathiesen, Improving pregnancy outcomes in women with diabetes mellitus: modern management. Nature Reviews Endocrinology, 2019. 15(7): p. 406-416.
19. Baptiste-Roberts, K., et al., Gestational diabetes and subsequent growth patterns of offspring: the National Collaborative Perinatal Project. Matern Child Health J, 2012. 16(1): p. 125-32.
20. Kc, K., S. Shakya, and H. Zhang, Gestational diabetes mellitus and macrosomia: a literature review. Ann Nutr Metab, 2015. 66 Suppl 2: p. 14-20.
21. Jährig, D., et al., Neonatal jaundice in infants of diabetic mothers. Acta Paediatr Scand Suppl, 1989. 360: p. 101-7.
22. Zhang, Y., et al., Maternal glycemic profiles during pregnancy and predelivery correlate with neonatal glucose homeostasis and jaundice risk: a prospective cohort study. Transl Pediatr, 2024. 13(11): p. 2012-2025.
23. Gewolb, I.H. and J. O'Brien, Surfactant secretion by type II pneumocytes is inhibited by high glucose concentrations. Exp Lung Res, 1997. 23(3): p. 245-55.
24. He, J., et al., Association between neonatal hyperbilirubinemia and hypoglycemia in Chinese women with diabetes in pregnancy and influence factors. Scientific Reports, 2022. 12(1): p. 16975.
25. Pantham, P., I.L. Aye, and T.L. Powell, Inflammation in maternal obesity and gestational diabetes mellitus. Placenta, 2015. 36(7): p. 709-15.
26. Feng, Z., et al., Abnormal neonatal brain microstructure in gestational diabetes mellitus revealed by MRI texture analysis. Scientific Reports, 2023. 13(1): p. 15720.
27. Ji, S., et al., Maternal hyperglycemia disturbs neocortical neurogenesis via epigenetic regulation in C57BL/6J mice. Cell Death Dis, 2019. 10(3): p. 211.
28. Ji, S., et al., Maternal hyperglycemia disturbs neocortical neurogenesis via epigenetic regulation in C57BL/6J mice. Cell Death & Disease, 2019. 10(3): p. 211.
29. Xiang, A.H., et al., Maternal Gestational Diabetes Mellitus, Type 1 Diabetes, and Type 2 Diabetes During Pregnancy and Risk of ADHD in Offspring. Diabetes Care, 2018. 41(12): p. 2502-2508.
30. Leach, L., Placental vascular dysfunction in diabetic pregnancies: intimations of fetal cardiovascular disease? Microcirculation, 2011. 18(4): p. 263-9.
31. Abell, S.K., et al., Inflammatory and Other Biomarkers: Role in Pathophysiology and Prediction of Gestational Diabetes Mellitus. Int J Mol Sci, 2015. 16(6): p. 13442-73.
32. Choi, S.H. and H.N. Ginsberg, Increased very low density lipoprotein (VLDL) secretion, hepatic steatosis, and insulin resistance. Trends Endocrinol Metab, 2011. 22(9): p. 353-63.
33. Higashi, Y., Endothelial Function in Dyslipidemia: Roles of LDL-Cholesterol, HDL-Cholesterol and Triglycerides. Cells, 2023. 12(9): p. 1293.
34. Possomato-Vieira, J.S. and R.A. Khalil, Mechanisms of Endothelial Dysfunction in Hypertensive Pregnancy and Preeclampsia. Adv Pharmacol, 2016. 77: p. 361-431.
35. Aziz, F., M.F. Khan, and A. Moiz, Gestational diabetes mellitus, hypertension, and dyslipidemia as the risk factors of preeclampsia. Sci Rep, 2024. 14(1): p. 6182.
36. Sullivan, S.D., J.G. Umans, and R. Ratner, Hypertension complicating diabetic pregnancies: pathophysiology, management, and controversies. J Clin Hypertens (Greenwich), 2011. 13(4): p. 275-84.
37. Olerich, K.L.W., et al., Cesarean delivery rates and indications in pregnancies complicated by diabetes. J Matern Fetal Neonatal Med, 2022. 35(26): p. 10375-10383.
38. Moon, J.H., et al., Multiparity increases the risk of diabetes by impairing the proliferative capacity of pancreatic β cells. Exp Mol Med, 2023. 55(10): p. 2269-2280.
